FREE Standard Shipping On All Orders $100 or More!*
Saltwater Pool Chemistry
Salt Water Generators or pool salt systems, create chlorine from electrolysis of a saline solution. In the process of making your own chlorine, chemical reactions occur that aren't present in tablet chlorinated pools.
The Topic of Discussion today is pH Rise, Corrosion and Cyanuric Acid; in a salt water pool.
pH Rise in a Saltwater Pool

In a salt pool, when electrolysis occurs (within the pipe), the products are hypochlorous acid, which is very acidic (as you may assume), and sodium hydroxide which is very basic. However, the acids and bases created by your salt cell will neutralize each other with very little net pH change.
The pH rise noticed by some salt chlorinator users may be a result of the outgassing of carbon dioxide, as water is agitated through the salt cell with hydrogen production.
A simpler answer is that Trichlor tablets, which have a very low pH, tends to suppress pH levels, or drive them lower over time, and in their absence, pH levels will naturally drift higher.
Corrosion in a Salt Pool
Salt is a corrosive substance, which is why winter road salt creates problems for concrete and steel.
Salt levels in a salt pool are usually quite low, under 3500 ppm. But even at low levels, galvanic corrosion can occur to stainless steel equipment (filters, lights, ladders) that is not properly bonded. Passive corrosion can affect soft porous stones and concrete around the pool.

What to do? One solution is pool equipment made of thermoplastics or bronze, or you can slow galvanic corrosion with a sacrificial anode. Zinc anodes attach to ladder, pool light or skimmer, and self-sacrifice, to draw corrosion away from other metal sources with lower electrochemical potential.
Cyanuric Acid in a Saltwater Pool
You didn't know that you have to use cyanuric acid (Stabilizer or Conditioner) in a salt pool? In fact, you should use more than pools that chlorinate with Trichlor tablets. Hayward and Pentair both recommend 60-80 ppm of cyanuric acid for their salt systems.
Why more CYA? Manufacturers of salt systems want more output from the salt cell, without fighting against UV degradation. As cyanuric acid bonds to chlorine, the chlorine residual can build faster.
However, when using high range CYA levels, above 50 ppm, you must also maintain a higher Free Chlorine level, in the range of 5 ppm, to compensate for the effects of cyanuric acid on chlorine.
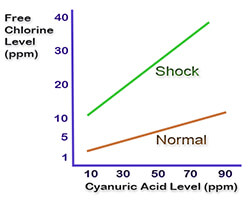
What effects? Cyanuric acid protects chlorine from the sun, but it also restricts chlorine activity; reduces kill rates and impacts ORP. As a result, many public pools are raising their FC minimum, for pools treated with cyanuric acid, or stabilizer. See an earlier blog post on chlorine kill rates in pools.
In a nutshell, water chemistry of salt water pools is not much different than tablet chlorine pools. Here's the key takeaways from the discussion.
- Maintain your pH at 7.4-7.6 and Total Alkalinity at 90-110 ppm
- Invest in a Sacrificial Anode for metal equipment or soft stones
- Test and Adjust Cyanuric Acid to manufacturer recommendation